Abstract
The antiphospholipid syndrome (APS) is characterized by thrombosis and/or specific pregnancy morbidity with the persistent presence of antiphospholipid antibodies (aPL). Given the high frequency of clinical symptoms irrespective of APS, laboratory tests are of utmost importance for the diagnosis of the disease. Laboratory criteria include aPL detection by a functional assay referred to as lupus anticoagulant (LAC) or by semi-quantitative solid phase assays measuring anti-β2glycoprotein I (aβ2GPI) or anti-cardiolipin (aCL) IgG/IgM antibodies. Reports from external quality control programs illustrate that commercially available aPL assays produce variable results, probably originating from differences in assay design, epitope exposure and a lack of standardization. In a large multicentre study, we aimed to assess the agreement between four solid phase assays measuring IgG and IgM aCL and aβ2GPI aPL.
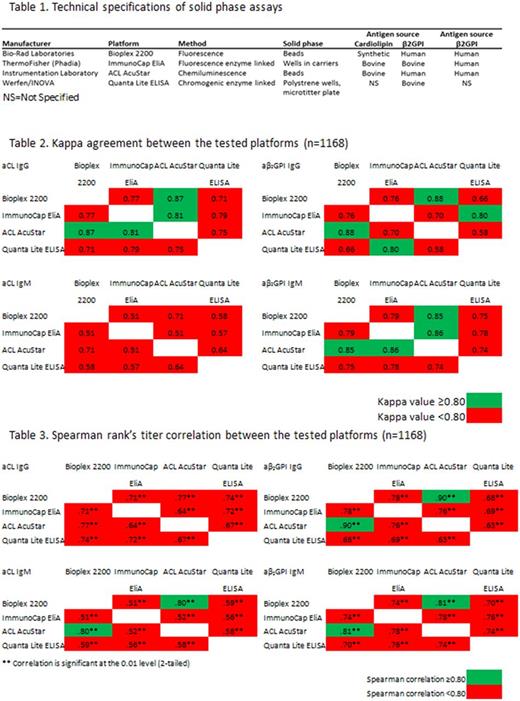
1168 patients were included from eight European centers. Patients were classified by the local centers resulting in 259 thrombotic APS patients, 204 diseased controls for thrombosis, 122 obstetric APS patients, 33 diseased controls for pregnancy complications, 196 patients with autoimmune disease other than APS, 100 pregnant women without complications, 194 patients without clinical and laboratory criteria for APS and 60 APS patients without specification of the clinical condition. Four platforms were selected based on frequency of use and the willingness of manufacturers to provide their assays (Table 1). All samples with all assays were tested at one location by a single technician.
To assess the agreement between the four platforms in the detection of IgG/IgM aCL and aβ2GPI aPL Kappa statistics was performed. A Kappa agreement value of 0.40-0.59 implies that only 15-35% of the data are reliable. Low levels of reliability affect diagnosis and treatment of APS patients and are unacceptable for clinical laboratories. In this study a Kappa agreement value of ≥0.8 ("good agreement") was considered as acceptable. The manufacturer's recommended cut-off value was used upon confirmation in at least 20 healthy donors.
Apart from Bioplex versus AcuStar and ImmunoCap EliA versus AcuStar (good agreement) all the other tested platforms did not reach a Kappa value ≥0.80 for aCL IgG and aβ2GPI IgM positivity (Table 2). Only two assays were in good agreement for aβ2GPI IgG positivity (AcuStar versus Bioplex and Quanta Lite ELISA versus ImmunoCap EliA), while all other tested platforms did not reach the threshold value. aCL IgM reactivity showed the lowest level of agreement, resulting in Kappa values <0.80 between the platforms.
Additionally, we determined the aPL titer correlation using a Spearman's rank test to be independent of differences in cut-off values. Values below the detection limit were replaced by the lower detection limit value. Apart from AcuStar and Bioplex all the other tested platforms had a Spearman's rank correlation coefficient <0.80 for aβ2GPI IgG, aCL and aβ2GPI IgM positivity (Table 3). None of the tested platforms resulted in a correlation coefficient ≥0.80 for aCL IgG.
To assess the clinical implications of the observed (dis)agreements, we investigated the number of patients that would be reclassified from thrombotic APS to non-APS thrombosis and vice versa based on our results. From the 259 thrombotic APS patients, 35 were negative for LAC (determined by the center). From these 35 patients, 18 up to 26 patients tested negative for any aPL in the four included platforms. Additionally, from the 204 non-APS thrombotic patients 1 up to 11 patients were positive for at least one aPL. In case of repetitive positivity, these patients in our hands would be classified as APS patients. Similar data were obtained for obstetric patients, resulting in a possible reclassification of 17 up to 21 patients.
Our study clearly indicates that diagnosis of APS is strongly platform dependent. Since APS patients have an increased risk for thrombosis recurrence, misclassification of patients has major implications. Falsely diagnosed patients may be given indefinite oral anticoagulant treatment, thus being exposed to a high risk of bleeding without having any benefit of such treatment. False-negative results have serious consequences as they need long-term anticoagulation to prevent recurrence. Standardization/uniformity of laboratory assays for the diagnosis of APS is therefore urgently warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal